Introduction
What is stem cell conditioned medium?
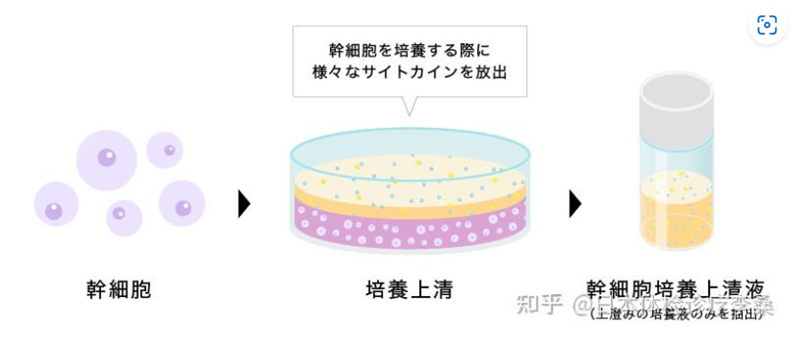
Stem cells derived from human umbilical cord, bone marrow, adipose tissue and other sources are cultured under controlled conditions. After the culturing process, the stem cells are removed, and the remaining culture medium is sterilized and further processed to produce stem cell-conditioned medium. We use advanced production technology to prepare a sterile, purified and concentrated Stem Cell Conditioned Media. All components of the purified concentrated Stem Cell Conditioned Media are of human origin and no animal-derived components are used in the production process. In addition, all stem cell donors are healthy volunteers with stringent virus and other tests by US CLIA (Clinical Laboratory Improvement Amendments)-certified laboratories, and all production processes are carried out in GMP workshops. We use independently developed purification methods to remove harmful substances as much as possible without removing growth factors and other beneficial substances
Composition and Efficacy
Stem cells are a very special type of cell that has the ability to self-repair and differentiate into various cell lineages. When stem cells grow and proliferate, they release proteins called ‘cytokines’. Through technical means, stem cells and other substances are removed altogether, so that a ‘Stem Cell Conditioned Medium’ concentrated with cytokines and growth factors can be prepared and stored at appropriate conditions. The product has a liquid or powder appearance and is transparent in color. It contains a variety of growth factors and can have a variety of beneficial effects, such as anti-inflammation, promoting angiogenesis, immune regulation, repairing nerve cells, beauty and skin care, etc. Representative substances and their functions are as follows:
1. EGF (Epidermal Growth Factor)
Promote epithelial cell growth, regeneration, and metabolism, inhibit melanocyte proliferation and so on.
2. FGF-1 (Fibroblast Growth Factor-1)
Promote the proliferation of epithelial cells and the formation of new blood vessels, and promote the synthesis of collagen, hyaluronic acid, SOD, etc.
3. FGF-2 (Fibroblast Growth Factor-2)
Promotes the growth and regeneration of the dermis and the proliferation of epidermal cells and muscle cells.
4. HGF (Hepatocyte Growth Factor)
Promote cell proliferation, tissue morphogenesis, inhibit cell apoptosis and proliferation of Maugham cell proliferation factors, etc.
Besides, we have conducted and are continuing to conduct multiple assessments in terms of anti-inflammation, anti-ageing functions, etc. Please enquire for more details.
Safety Profiles
Regarding the stem cell conditioned medium independently developed by our company, we have undergone strict donor screening and safety testing to ensure the high purity and safety of our products.
Donor virus testing
The results of confirmation tests for HIV, TP, HAV, HBV, HCV, CMV, etc. were all negative.
Conditioned medium virus detection
The results of confirmation tests for HIV, HBV、HCV, CMV, EB etc. were all negative.
Mycoplasma detection
The result is negative.
Sterility test
The product is confirmed to be sterile.
Endotoxin detection
Endotoxin concentration ≤0.3EU/ml.
Other safety testing
We have conducted and are continuing to conduct multiple safety assessments in terms of inflammation induction, dermis destruction, etc. Please enquire for more details.

aCGT’s Product
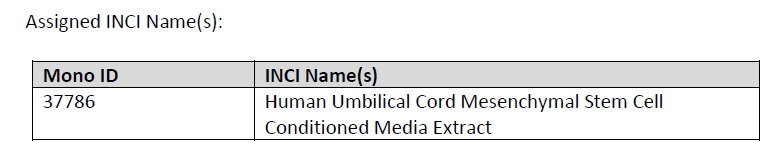
aCGT has obtained INCI (International Nomenclature of Cosmetic Ingredients) designation for our Stem Cell Conditioned Media from the Personal Care Products Council (PCPC).
The designation qualifies our Stem Cell Conditioned Media as a raw material for cosmetic purposes. We can distribute our product via our Hong Kong center. If you have any question, please ENQUIRE.
What is INCI?
INCI names (International Nomenclature Cosmetic Ingredient) are systematic names internationally recognized to identify cosmetic ingredients. The International Nomenclature Committee (INC) develops them, and the Personal Care Products Council (PCPC) publishes them in the International Cosmetic Ingredient Dictionary and Handbook, which is also available electronically as wINCI.
Oversight for the INCI program is provided by PCPC as part of its mission to support the identification of the composition of personal care products, and publication of this information in a worldwide science-based Dictionary. PCPC is committed to ensuring that the Dictionary provides the world community with accurate, transparent, and harmonized nomenclature. By working closely with its international sister trade associations, and with other organizations around the world, PCPC strives to develop INCI names that accommodate differing labeling approaches described in national laws and regulations.